- Latest news▼
-
12:16, April 19 Scientists grow human mini-lungs in lab

-
10:23, April 19 JAMA Oncology: Urine test can help rule out high-grade prostate cancer with almost 100% accuracy, study shows

-
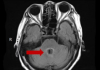
18:00, April 18 Daily Mail: Elderly woman in China gets infected with brain-eating amoeba
-
14:19, April 18 Obesity: exercising before breakfast helps you lose weight faster

-
10:42, April 18 The Conversation: childhood trauma can cause pathological hoarding

-
08:37, April 18 Daily Mail: Satiating food reduces cravings for sweets, nutritionist says

-
18:22, April 17 First Armenian-German Conference entitled “Heart Failure Spring School”

-
08:38, April 17 Why do kids usually recover from COVID-19 more easily than adults?

-
14:37, April 16 Daily Mail: intermittent fasting is not suitable for children and women before their periods

-
16:41, April 15 Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk

-
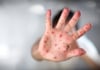
15:04, April 15 305 cases of measles recorded in Armenia so far in 2024
-
14:38, April 15 Food and Environmental Virology: tea contributes to effective coronavirus control

-
12:41, April 15 Daily Mail: vitamin A, B3 and E supplements can be dangerous
-
10:56, April 15 Diabetes Care: evening physical activity is good for the heart

-
08:27, April 15 Women are more susceptible to blood loss and death during bypass surgery than men, researchers say
All materials
mTOR inhibitors to combat drug-resistant tumors
Howard Hughes Medical Institute scientists have designed a potential cancer therapy that uses a unique strategy to block mTOR, a molecule that helps drive the growth of many tumors. In animal experiments, the drug reduces the size of tumors that are resistant to earlier-generation mTOR inhibitors.
{"uid":1,"hostPeerName":"http://medicalxpress.com","initialGeometry":"{\"windowCoords_t\":0,\"windowCoords_r\":1349,\"windowCoords_b\":667,\"windowCoords_l\":0,\"frameCoords_t\":817.546875,\"frameCoords_r\":386.703125,\"frameCoords_b\":1067.546875,\"frameCoords_l\":86.703125,\"styleZIndex\":\"auto\",\"allowedExpansion_t\":0,\"allowedExpansion_r\":0,\"allowedExpansion_b\":0,\"allowedExpansion_l\":0,\"xInView\":0,\"yInView\":0}","permissions":"{\"expandByOverlay\":false,\"expandByPush\":false,\"readCookie\":false,\"writeCookie\":false}","metadata":"{\"shared\":{\"sf_ver\":\"1-0-2\",\"ck_on\":1,\"flash_ver\":\"21.0.0\"}}","reportCreativeGeometry":false}" scrolling="no" marginwidth="0" marginheight="0" width="300" height="250" data-is-safeframe="true" style="border-bottom-width:0px;border-left-width:0px; border-right-width:0px;border-top-width:0px;font-family:sans-serif;margin-bottom: 0px;margin-left:0px;margin-right:0px;margin-top:0px;padding-bottom:0px; padding-left:0px;padding-right:0px;padding-top:0px;vertical-align:bottom"> The work, reported May 18, 2016, in the journal Nature, was led by HHMI investigator Kevan Shokat at the University of California, San Francisco and Neal Rosen at Memorial Sloan Kettering Cancer Center.
Several anti-cancer drugs attempt to halt the growth of tumors by blocking mTOR, a key part of a growth-regulating network that is often disrupted in cancer cells. The oldest of these drugs, rapamycin and related molecules called rapalogs, have had some success in treating a few types of cancer, including kidney and breast cancers. Second-generation mTOR inhibitors, designed to block mTOR signaling more potently than rapalogs, are currently being evaluated in clinical trials.
Unfortunately, tumors can become resistant to rapalogs after months or years of effective treatment—and it's likely resistance will develop to second-generation mTOR inhibitors, too. It's a common problem with molecularly targeted cancer therapies, Shokat says. But if researchers understand the changes that make cancer cells resistant to a drug, they can work to develop a next-generation therapy.
Shokat and his colleagues wanted to get ahead of this problem, and began thinking about third-generation mTOR inhibitors without waiting for patients to develop resistance to the latest therapies. To anticipate how resistance might arise, Rosen's team exposed breast cancer-derived cells that they grew in the lab to either rapamycin or to a second-generation mTOR inhibitor, ADZ8055, for three months. Most of the cells died, but as expected, some found a way to survive and multiply.
The researchers examined those drug-resistant cells to identify the specific changes that had allowed them to thrive, reasoning that the same changes might arise in patients who received the drugs. Then Rosen's team searched for the mutations in genetic databases that catalogued tumors from cancer patients.
To their surprise, the mutations that had made their lab-grown cells resistant to ADZ8055 were present in some patients' tumors even before treatment. "That really was a shock, because usually those are drug-induced mutations," Shokat says. The changes, present in about 10 percent of renal cell tumors (the most common type of kidney cancer), boosted mTOR activity, which meant they aided tumor growth with or without ADZ8055.
Patients whose tumors carried the mutations would never respond to the second-generation mTOR inhibitor, Shokat says. "Immediately, the project changed from 'down the road patients are going to have these mutations, so we're going to need a drug' to 'wow, patients already have these mutations, and they are de novo resistant to the drug we have in the clinic.'"
The researchers knew they needed an mTOR inhibitor that worked differently than its predecessors. To design one, Shokat took inspiration from the immune system. Antibodies excel at binding to rapidly changing targets, thanks to their Y-shaped structure with two antigen-binding tips. The two binding sites give antibodies strong affinity for their targets, Shokat says, and he wanted to mimic that strategy by creating an inhibitor that bound to mTOR in two places.
He did this by linking a first-generation mTOR inhibitor, which binds to one part of the molecule, to a second-generation inhibitor, which targets a separate pocket nearby. The new inhibitor, called Rapalink, grabs onto mTOR in both spots, and binds better than earlier-generation inhibitors.
What's more, the two-pronged inhibitor can grasp mTOR even if it contains mutations that prevent simpler inhibitors from binding. That's because it's not essential that both mTOR binding sites are a perfect match for the drug. Shokat explains: Once one side of the inhibitor has latched on to its target, its other end is tethered close to its own binding pocket and thus likely latch on, too.
The scientists showed that Rapalink can get inside cancer cells and shut off mTOR, even if those cells are resistant to earlier generation inhibitors. They also tested Rapalink's ability to inhibit tumor growth in animal experiments, and found that it reduced the size of tumors that were resistant to first- or second-generation mTOR inhibitors. "It worked like a charm," Shokat says.
The scientists have licensed Rapalink to Kura Oncology, which will continue to evaluate its potential as a cancer therapy. Shokat and Rosen are scientific advisors to Kura Oncology.
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Archive
- Most read
month
week
day
- WHO: Nigeria pioneers revolutionary meningitis vaccine 1178
- One-third of women experience menstruation-related migraines, most often during premenopause - study 1152
- Daily Mail: vitamin A, B3 and E supplements can be dangerous 989
- Food and Environmental Virology: tea contributes to effective coronavirus control 985
- Women are more susceptible to blood loss and death during bypass surgery than men, researchers say 960
- Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk 957
- 305 cases of measles recorded in Armenia so far in 2024 949
- Diabetes Care: evening physical activity is good for the heart 919
- Daily Mail: intermittent fasting is not suitable for children and women before their periods 766
- First Armenian-German Conference entitled “Heart Failure Spring School” 551
- The Conversation: childhood trauma can cause pathological hoarding 430
- Why do kids usually recover from COVID-19 more easily than adults? 430
- Obesity: exercising before breakfast helps you lose weight faster 429
- Daily Mail: Elderly woman in China gets infected with brain-eating amoeba 392
- Daily Mail: Satiating food reduces cravings for sweets, nutritionist says 388
- Find us on Facebook
- Poll





