- Latest news▼
-
12:16, April 19 Scientists grow human mini-lungs in lab

-
10:23, April 19 JAMA Oncology: Urine test can help rule out high-grade prostate cancer with almost 100% accuracy, study shows

-
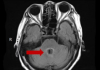
18:00, April 18 Daily Mail: Elderly woman in China gets infected with brain-eating amoeba
-
14:19, April 18 Obesity: exercising before breakfast helps you lose weight faster

-
10:42, April 18 The Conversation: childhood trauma can cause pathological hoarding

-
08:37, April 18 Daily Mail: Satiating food reduces cravings for sweets, nutritionist says

-
18:22, April 17 First Armenian-German Conference entitled “Heart Failure Spring School”

-
08:38, April 17 Why do kids usually recover from COVID-19 more easily than adults?

-
14:37, April 16 Daily Mail: intermittent fasting is not suitable for children and women before their periods

-
16:41, April 15 Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk

-
15:04, April 15 305 cases of measles recorded in Armenia so far in 2024
-
14:38, April 15 Food and Environmental Virology: tea contributes to effective coronavirus control

-
12:41, April 15 Daily Mail: vitamin A, B3 and E supplements can be dangerous
-
10:56, April 15 Diabetes Care: evening physical activity is good for the heart

-
08:27, April 15 Women are more susceptible to blood loss and death during bypass surgery than men, researchers say
All materials
Cells genetically modified to produce insulin could transform diabetes treatment

Genetically modified cells could eliminate the need for daily injections to treat type 1 diabetes, experts have revealed.
The human cell line is genetically engineered to produce, store and release insulin in response to blood sugar levels in the human body.
Scientists said the insulin-producing 'Melligen' cells show promise as a possible cure for type 1 diabetes.
In type 1 diabetes, the immune system destroys the pancreatic cells responsible for making insulin, a hormone crucial to converting blood sugar into energy.
This month, they secured US patent protection for the cell line from the US Patent and Trademark Office.
Professor Ann Simpson, who led the team at the University of Technology, Sydney, said: 'My team and I are extremely pleased that the US patent for the Melligen cells has been granted.
'This takes us a step closer to releasing diabetics from the need to inject insulin daily, and more importantly, protecting them from the debilitating complications of the disease, such as blindness, kidney failure and cardiovascular problems.'
The researchers are now working with US clinical biotech firm PharmaCyte Biotech to develop the research into new treatments.
PharmaCyte specialises in the development of targeted treatments for cancer and diabetes using its signature live cell encapsulation technology.
This technology, known as Cell-in-a-Box, is a key process in the commercialisation of the Melligen cell as a revolutionary treatment.
'This is a culmination of many years' work by our group and we look forward to working with PharmaCyte's Diabetes Consortium to utilise the Cell-in–a-Box technology to encapsulate the cells for preclinical trials aimed at curing diabetes,' said Professor Simpson.
'We anticipate that the capsule technology will protect the Melligen cells from the body's immune response that normally destroys foreign tissue, allowing the Melligen cells to be transplanted into humans.'
PharmaCyte's chief executive officer, Kenneth Waggoner, said: 'We at PharmaCyte consider ourselves to be very fortunate in having secured the exclusive world-wide licence to use the Melligen cells to develop a treatment for diabetes.
'If we are successful in this effort, it will bring to fruition the many years of research that have been conducted by Professor Simpson and her colleagues at UTS in developing these remarkable cells.
'Importantly, we are very pleased that Professor Simpson will be assisting PharmaCyte in this endeavour as a member of our International Diabetes Consortium.
'For the millions of people worldwide who suffer from a disease of epidemic proportions, our treatment could relieve them of the onerous daily requirements for insulin administration and dietary restrictions and offer a life free from the very serious and even life-threatening complications associated with diabetes.'
With the World Health Organization attributing more than 1.5 million deaths to diabetes in 2012 and more than 422 million adults suffering from the disease in 2014, the development has the potential to impact millions of lives.
The cell line already has patent protection from the European counterpart to the US Patent and Trademark Office and the patent has been validated in France, Switzerland, Great Britain, Ireland, Germany, Spain, Denmark, Italy and the Netherlands.
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Archive
- Most read
month
week
day
- WHO: Nigeria pioneers revolutionary meningitis vaccine 1184
- One-third of women experience menstruation-related migraines, most often during premenopause - study 1171
- Daily Mail: vitamin A, B3 and E supplements can be dangerous 1024
- Food and Environmental Virology: tea contributes to effective coronavirus control 1016
- Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk 989
- 305 cases of measles recorded in Armenia so far in 2024 981
- Women are more susceptible to blood loss and death during bypass surgery than men, researchers say 969
- Diabetes Care: evening physical activity is good for the heart 925
- Daily Mail: intermittent fasting is not suitable for children and women before their periods 799
- First Armenian-German Conference entitled “Heart Failure Spring School” 580
- Why do kids usually recover from COVID-19 more easily than adults? 466
- Obesity: exercising before breakfast helps you lose weight faster 465
- The Conversation: childhood trauma can cause pathological hoarding 462
- Daily Mail: Elderly woman in China gets infected with brain-eating amoeba 441
- Daily Mail: Satiating food reduces cravings for sweets, nutritionist says 436
- Find us on Facebook
- Poll





