- Latest news▼
-
10:23, April 19 JAMA Oncology: Urine test can help rule out high-grade prostate cancer with almost 100% accuracy, study shows

-
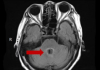
18:00, April 18 Daily Mail: Elderly woman in China gets infected with brain-eating amoeba
-
14:19, April 18 Obesity: exercising before breakfast helps you lose weight faster

-
10:42, April 18 The Conversation: childhood trauma can cause pathological hoarding

-
08:37, April 18 Daily Mail: Satiating food reduces cravings for sweets, nutritionist says

-
18:22, April 17 First Armenian-German Conference entitled “Heart Failure Spring School”

-
08:38, April 17 Why do kids usually recover from COVID-19 more easily than adults?

-
14:37, April 16 Daily Mail: intermittent fasting is not suitable for children and women before their periods

-
16:41, April 15 Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk

-
15:04, April 15 305 cases of measles recorded in Armenia so far in 2024
-
14:38, April 15 Food and Environmental Virology: tea contributes to effective coronavirus control

-
12:41, April 15 Daily Mail: vitamin A, B3 and E supplements can be dangerous
-
10:56, April 15 Diabetes Care: evening physical activity is good for the heart

-
08:27, April 15 Women are more susceptible to blood loss and death during bypass surgery than men, researchers say
-
18:42, April 13 WHO: Nigeria pioneers revolutionary meningitis vaccine
All materials
A ‘Milestone’ In Fighting Influenza: FDA Approves US Facility To Manufacture Cell-Based Flu Vaccine
The U.S. Department of Health and Human Services (HHS) has announced a “milestone” in fighting influenza: a U.S. facility has been approved by the Food & Drug Administration (FDA) to manufacture vaccines using a cell-based technology, Medical Daily reports.
Though a cell-based flu vaccine named Flucelvax was approved by the FDA in 2012, the approval of this particular facility would increase the capacity for seasonal influenza vaccine production “by at least 50 million doses,” an HHS statement reads.
Cell-based flu vaccines, or cell culture technology, are an alternative to the traditional egg-based process used to make regular flu vaccines. They are produced by growing viruses in animal cells. The main difference between them and regular flu vaccines is that the influenza A and B viruses are grown in mammalian cells rather than hens’ eggs. According to the Centers for Disease Control and Prevention (CDC), cell-based flu vaccines are being developed due to a higher level of flexibility over egg-based vaccines. This process would also provide a faster way to develop vaccines in the case of a flu pandemic, as the cells used are frozen and stored to ensure availability.
The cell-based technology that has been approved is located at a facility in Holly Springs, North Carolina. The FDA’s approval of Flucelvax in 2012 allowed this technique to be used for the first time throughout the U.S., though it’s been available in various European countries for some time. Now, the Novartis Holly Springs facility will be the first in the U.S. to manufacture Flucelvax
Cell-culture technology is the first major advancement in influenza vaccine production in the US in more than 40 years. Cell-based vaccines have been used for polio, rotavirus, hepatitis, smallpox, rubella and chickenpox.
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Archive
- Most read
month
week
day
- Pediatrics: Hypoglossal nerve stimulation implant helps with sleep apnea 1362
- WHO: Nigeria pioneers revolutionary meningitis vaccine 1170
- One-third of women experience menstruation-related migraines, most often during premenopause - study 1136
- Women are more susceptible to blood loss and death during bypass surgery than men, researchers say 952
- Food and Environmental Virology: tea contributes to effective coronavirus control 934
- Daily Mail: vitamin A, B3 and E supplements can be dangerous 933
- Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk 905
- 305 cases of measles recorded in Armenia so far in 2024 897
- Diabetes Care: evening physical activity is good for the heart 887
- Daily Mail: intermittent fasting is not suitable for children and women before their periods 710
- First Armenian-German Conference entitled “Heart Failure Spring School” 504
- Why do kids usually recover from COVID-19 more easily than adults? 375
- Obesity: exercising before breakfast helps you lose weight faster 362
- The Conversation: childhood trauma can cause pathological hoarding 354
- Daily Mail: Satiating food reduces cravings for sweets, nutritionist says 328
- Find us on Facebook
- Poll





