- Latest news▼
-
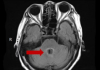
18:00, April 18 Daily Mail: Elderly woman in China gets infected with brain-eating amoeba
-
14:19, April 18 Obesity: exercising before breakfast helps you lose weight faster

-
10:42, April 18 The Conversation: childhood trauma can cause pathological hoarding

-
08:37, April 18 Daily Mail: Satiating food reduces cravings for sweets, nutritionist says

-
18:22, April 17 First Armenian-German Conference entitled “Heart Failure Spring School”

-
08:38, April 17 Why do kids usually recover from COVID-19 more easily than adults?

-
14:37, April 16 Daily Mail: intermittent fasting is not suitable for children and women before their periods

-
16:41, April 15 Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk

-
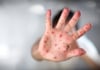
15:04, April 15 305 cases of measles recorded in Armenia so far in 2024
-
14:38, April 15 Food and Environmental Virology: tea contributes to effective coronavirus control

-
12:41, April 15 Daily Mail: vitamin A, B3 and E supplements can be dangerous
-
10:56, April 15 Diabetes Care: evening physical activity is good for the heart

-
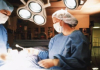
08:27, April 15 Women are more susceptible to blood loss and death during bypass surgery than men, researchers say
-
18:42, April 13 WHO: Nigeria pioneers revolutionary meningitis vaccine
-
16:43, April 13 One-third of women experience menstruation-related migraines, most often during premenopause - study

All materials
FDA ticks off first drug to treat radiation sickness after nuclear disasters

A drug long-used to counter the negative effects of chemotherapy has won US Food and Drug Administration (FDA) approval for use in treating the nasty effects of exposure to radiation following a nuclear disaster. Known commercially as Neupogen, the drug has been shown to work by shielding the body's white blood cells to heighten a patient's chances of survival.
Neupogen, or filgrastim as it is otherwise known, is a synthetic protein that boosts the growth of infection-fighting white blood cells. Where the production of these cells is hampered in cancer patients by chemotherapy and radiation therapy, Neupogen can be used to stimulate the growth, maturation and release of white cells from the bone marrow. This better equips the sufferer to ward off infections and bleeding problems that can result from the therapy.
Neupogen was first approved for helping to treat those undergoing chemotherapy in 1991, and has since been one of a number of multi-purpose drugs investigated for potential use in the aftermath of nuclear disasters. But research conducted at the University of Maryland has now uncovered evidence worthy of the FDA's nod, making Neupogen the first drug to be approved as a countermeasure for Hematopoietic Acute Radiation Syndrome (H-ARS).
The scientists carried out their study in a non-human clinical model of high-dose radiation, with the FDA saying that, in the absence of ethical human studies, these animal studies were adequate and well enough controlled to suggest Neupogen is reasonably likely to be of benefit to humans suffering from H-ARS.
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Archive
- Most read
month
week
day
- Pediatrics: Hypoglossal nerve stimulation implant helps with sleep apnea 1361
- Health minister: Simulation educational center will be created, assisted reproductive technology capacity will increase in Armenia 1317
- WHO: Nigeria pioneers revolutionary meningitis vaccine 1169
- One-third of women experience menstruation-related migraines, most often during premenopause - study 1136
- Women are more susceptible to blood loss and death during bypass surgery than men, researchers say 945
- Daily Mail: vitamin A, B3 and E supplements can be dangerous 922
- Food and Environmental Virology: tea contributes to effective coronavirus control 922
- Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk 894
- 305 cases of measles recorded in Armenia so far in 2024 886
- Diabetes Care: evening physical activity is good for the heart 876
- Daily Mail: intermittent fasting is not suitable for children and women before their periods 700
- First Armenian-German Conference entitled “Heart Failure Spring School” 493
- Why do kids usually recover from COVID-19 more easily than adults? 362
- Obesity: exercising before breakfast helps you lose weight faster 347
- The Conversation: childhood trauma can cause pathological hoarding 340
- Find us on Facebook
- Poll





