- Latest news▼
-
19:41, April 25 Children’s Hospital Los Angeles and International Center of Professional Development Allergy/Immunology Conference

-
17:31, April 25 JAMA: patient grew long, curly eyelashes because of chemotherapy
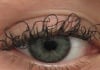
-
11:08, April 25 Mpox epidemic declared in Republic of the Congo

-
08:31, April 25 OU: quitting smoking 4 times more likely to cure laryngeal cancer

-
01:20, April 25 Paralyzed man in China writes hieroglyphs using neural implants placed in his brain

-
15:11, April 24 Zombie deer disease possibly linked to hunters’ deaths

-
12:27, April 23 Appetite: Scientists found out the secret to the appeal of large portions of fast food

-
10:33, April 23 Scientists test new approach to fighting viruses

-
08:38, April 23 Ketamine may help with postpartum depression

-
22:12, April 22 Unhealthy amount of sugar found in baby food products of a well-known brand

-
19:41, April 22 Air pollution puts health of more than 1.6 billion workers globally at risk

-
17:25, April 22 Scientists found baked goods and lack of sleep to be more dangerous than alcohol

-
16:02, April 22 342 cases of measles recorded in Armenia so far in 2024
-
15:29, April 22 BrainStimulation: electrical brain stimulation alleviates anxiety and depression in the elderly

-
08:27, April 22 Cognitively stimulating jobs in midlife could lower dementia risk in old age, study finds

All materials
Seventeen volunteers let this worm live inside them to help defeat a dangerous disease
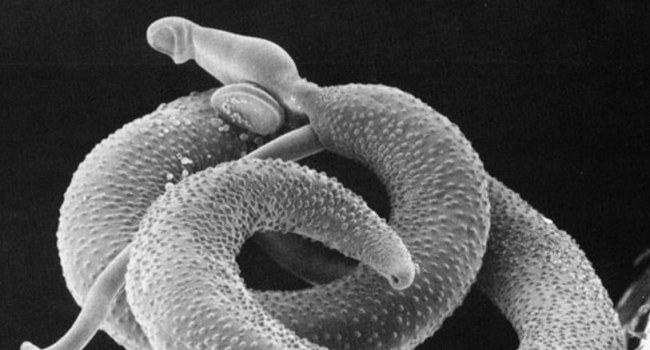
At 12:05 p.m. on a Thursday in February, a lab technician takes a six-well plate containing a solitary red snail and places it in a heated water bath under a strong light. The light and warmth signal hundreds of tiny larval parasites to stream out of the mollusk. Now, the clock starts ticking for Meta Roestenberg, an infectious disease physician here at Leiden University Medical Center. She has about 4 hours to launch a unique, controversial experiment in which she will let the parasites burrow into the arms of four healthy volunteers. If she waits too long, the larvae start to die.
Roestenberg and her colleagues are infecting people with Schistosoma mansoni, one of five tiny waterborne worm species that cause schistosomiasis, a disease that sickens millions of people in Africa, the Middle East, and Latin America and kills thousands each year. There is no schistosomiasis vaccine and only one old, inadequate drug, praziquantel, to treat it. Infecting humans could help speed up the development of new interventions. Roestenberg has designed the experiment to prevent the parasites from reproducing, and she says the risk to volunteers is extremely low.
But not low enough, some scientists argue, because there is no guarantee that subjects will get rid of their parasites when the study is over. “I would not volunteer for this study and if I had a son or daughter who wanted to volunteer, I would recommend against it,” says Daniel Colley, a schistosomiasis researcher at the University of Georgia in Athens.
At 1:05 p.m., the technician takes the plate out of the bath. The larvae are ready to be harvested. Viewed under a microscope, they move around frantically, like minipropellers. Another technician removes one drop, dilutes it, adds iodine to kill the parasites, and counts them. That allows the researchers to calculate how many are left in the well: 574. They need only 80 today, 20 per volunteer.
A snail population in an African lake could shed millions of these larvae into the water on a single day, each equipped with a chemical sensor that lets it home in on humans entering the water. After penetrating the skin, they migrate to the liver, where they mature and mate. Male-female couples stay together and move to blood vessels in the bowel, where they can reside for years, shedding hundreds of eggs a day. Most eggs end up in urine and feces, and if they make their way back into the lake they may infect fresh snails. But some get trapped in the liver, kidneys, or spleen, causing damage and leading to pain, blood loss, malnutrition, and sometimes death.
Researchers in this same lab recreated the parasite’s life cycle decades ago, with hamsters taking the place of humans. That allowed them to produce and study S. mansoni. Now, Roestenberg wants to bring humans back into the mix. Field trials, especially of vaccines, are hugely expensive and complex, and the risk of failure is considerable. A controlled infection study can act as a gatekeeper, she says: “It gives you an indication whether something can work in humans or not.”
Researchers have long grown Schistosoma mansoni in the lab, using hamsters. Now, they are also infecting humans with the parasitic worms.
Studies in which people are purposely infected with malaria, cholera, and flu are on the rise, but they haven’t been done with schistosomiasis, in part because damage from the S. mansoni eggs can be irreversible. The goal of the current study, which began in early 2017, is to find out whether Roestenberg’s infection model is safe; if so, she hopes to test a vaccine later this year.
At 1:35 p.m., Roestenberg walks to the room where the volunteers will be infected. She opens a transparent plastic container that contains epinephrine, antihistamines, and corticosteroids. “This is the emergency box,” she says—in case a subject has a strong allergic reaction. None of the 13 volunteers infected so far has, although one who was infected with 30 larvae developed a strong fever. In another precaution, the volunteers have been tested to rule out risk factors such as HIV infection and pregnancy. In nature, people become infected with both male and female parasites, but Roestenberg uses only males, so there will be no eggs and thus, she says, no symptoms. And when the study ends in 12 weeks, the volunteers will be given praziquantel to cure them.
That drug, Colley emphasizes, is “not terribly effective.” But Roestenberg says that even if it fails, volunteers needn’t worry. “The ethics board asked me: ‘If one worm survives even after multiple treatments, what will happen to that person?’ And I said: ‘They’ll probably live to be 100.’” The board gave her its blessing. Colley agrees the risk is low, but still, S. mansoni has an average life span of 5 to 10 years. “That is a long time to have something as ugly as a schistosome living in your blood vessels, putting out excrement and things.”
At 2:15 p.m., Roestenberg huddles in a small meeting room with three colleagues. The worms are not drugs, but they need to be released for use just like an experimental drug would be. The scientists check numbers on some documents against data on a computer screen, then they sign a form. The experiment can begin.
Twenty minutes later, back in the infection room, the volunteers stretch out their arms so that a little metal cylinder, a few centimeters in diameter, can be taped to their skin. Carefully, an assistant pipettes a few drops of water, containing exactly 20 parasites, into each cylinder. The volunteers are nervous, but they say they are motivated. “I like the fact that the study is related to vaccines, because I’ve worked in that field before,” says one, a young scientist. The woman next to him says she comes from East Africa and knows the disease firsthand. They will also be paid €1000 for their time.
Once infected, the volunteers will return to the lab every week so the research team can test their blood for a molecule called CAA, which the worms regurgitate from their stomachs. CAA’s presence will indicate that the worms are still alive; in future trials, its absence might mean that a vaccine or drug has worked.
Some schistosomiasis scientists agree that the potential benefits justify the minimal risks. “My hope is that it would hugely accelerate identification of worthwhile candidate vaccines,” says Alison Elliott of the London School of Hygiene & Tropical Medicine, who works at a joint Ugandan and U.K. research unit in Entebbe. She is interested in establishing the model there; people in Uganda, a country badly affected by schistosomiasis, might react differently to a vaccine if they were exposed to the worms in childhood, she explains. At a recent stakeholders’ meeting, “Ethics and regulatory colleagues were very supportive of taking discussions of the model forward, and community representatives are already keen for the opportunity to volunteer!” Elliott added in an email.
“It’s itching a little bit,” one of the Leiden volunteers says 5 minutes into the exposure. After half an hour, when the infested water is removed from the volunteers’ forearms, red spots reveal where the parasites have entered their new hosts. Then, close to 4 p.m., the clock stops ticking; the volunteers head home and Roestenberg and her colleagues go out for a coffee.
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Children’s Hospital Los Angeles and International Center of Professional Development Allergy/Immunology Conference
- First Armenian-German Conference entitled “Heart Failure Spring School”
- Allogeneic bone marrow transplant in case of hematological malignancy performed in Armenia for first time
All materials
- Archive
- Most read
month
week
day
- JAMA Oncology: Urine test can help rule out high-grade prostate cancer with almost 100% accuracy, study shows 1273
- Scientists grow human mini-lungs in lab 1157
- Next pandemic likely to be triggered by flu - scientists 920
- Scientists found baked goods and lack of sleep to be more dangerous than alcohol 847
- 342 cases of measles recorded in Armenia so far in 2024 804
- Blood test can determine who is at risk of developing multiple sclerosis - scientists 765
- Scientists develop new method to safely stimulate immune cells to fight cancer 764
- Cognitively stimulating jobs in midlife could lower dementia risk in old age, study finds 758
- BrainStimulation: electrical brain stimulation alleviates anxiety and depression in the elderly 706
- Unhealthy amount of sugar found in baby food products of a well-known brand 585
- Air pollution puts health of more than 1.6 billion workers globally at risk 581
- Ketamine may help with postpartum depression 569
- Appetite: Scientists found out the secret to the appeal of large portions of fast food 567
- Scientists test new approach to fighting viruses 551
- Zombie deer disease possibly linked to hunters’ deaths 447
- Find us on Facebook
- Poll





