- Latest news▼
-
12:16, April 19 Scientists grow human mini-lungs in lab

-
10:23, April 19 JAMA Oncology: Urine test can help rule out high-grade prostate cancer with almost 100% accuracy, study shows

-
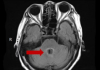
18:00, April 18 Daily Mail: Elderly woman in China gets infected with brain-eating amoeba
-
14:19, April 18 Obesity: exercising before breakfast helps you lose weight faster

-
10:42, April 18 The Conversation: childhood trauma can cause pathological hoarding

-
08:37, April 18 Daily Mail: Satiating food reduces cravings for sweets, nutritionist says

-
18:22, April 17 First Armenian-German Conference entitled “Heart Failure Spring School”

-
08:38, April 17 Why do kids usually recover from COVID-19 more easily than adults?

-
14:37, April 16 Daily Mail: intermittent fasting is not suitable for children and women before their periods

-
16:41, April 15 Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk

-
15:04, April 15 305 cases of measles recorded in Armenia so far in 2024
-
14:38, April 15 Food and Environmental Virology: tea contributes to effective coronavirus control

-
12:41, April 15 Daily Mail: vitamin A, B3 and E supplements can be dangerous
-
10:56, April 15 Diabetes Care: evening physical activity is good for the heart

-
08:27, April 15 Women are more susceptible to blood loss and death during bypass surgery than men, researchers say
All materials
Female XX sex chromosomes increase survival and extend lifespan in aging mice

Female longevity is observed in humans and much of the animal kingdom, but its causes remain elusive. Using a genetic manipulation that generates XX and XY mice, each with either ovaries or testes, we show that the female XX sex chromosome complement increases survival during aging in male and female mice. In combination with ovaries, it also extends lifespan.
Understanding causes of sex‐based differences in aging could lead to new pathways to counter age‐induced decline in both sexes.
Women live longer than men around the world, regardless of culture or socioeconomic status (UnitedNations, 2015; Zarulli et al., 2018). Female longevity is also observed in the animal kingdom (Barrett & Richardson, 2011; Bronikowski et al., 2011; Clutton‐Brock & Isvaran, 2007) due to causes that may be extrinsic, intrinsic, or both. Extrinsic causes of sex difference in invertebrates can signal antagonistic survival strategies: female pheromones reduce male lifespan in Drosophila (Gendron et al., 2014), and male secretions shorten hermaphrodite lifespan in C. elegans (Maures et al., 2014).
Intrinsic effects—operating within the organism—underlie longer life in organisms following removal of reproductive cells or organs in C. elegans hermaphrodites (Berman & Kenyon, 2006), male and female dogs (Hoffman, Creevy, & Promislow, 2013), and possibly men as suggested by a study of eunuchs (Min, Lee, & Park, 2012). Nonetheless, causes of intrinsic sex difference in lifespan remain largely unknown. The pervasive nature of female longevity in humans, even in early death during severe epidemics and famine (Zarulli et al., 2018), suggests a role for innate biology in the survival gap between the sexes. Here, we sought to identify intrinsic causes of female longevity in mammalian lifespan.
Sex chromosomes or gonads cause intrinsic sex differences in mammals, but whether they directly contribute to increased female lifespan is unknown in mammalian aging. To dissect these etiologies, we used four core genotypes (FCG) mice (Arnold, 2004). In mice and humans, the Sry gene normally resides on the Y chromosome and codes for a protein (testicular determining Y factor) that induces development of testes and perinatal masculinization. In FCG mice, Sry resides instead on an autosome, enabling inheritance of Sry—and thus male, testicular phenotype—with or without the Y chromosome.
The genetic manipulation of SRY generates XX and XY mice, each with either ovaries (O) or testes (T): XX(O), XX(T), XY(O), XY(T). Gonadal hormone levels in FCG mice with the same gonads are comparable, regardless of their sex chromosomes (Gatewood et al., 2006; McCullough et al., 2016). In FCG model mice, a sex difference with a main effect that statistically differs by genotype (XX vs. XY) is sex chromosome‐mediated; one that differs by phenotype (ovaries vs. testes) is gonadal sex‐mediated. Examples of age‐relevant FCG mouse studies show that XX improves blood pressure regulation (Pessoa et al., 2015) and attenuates experimental brain injuries (Du et al., 2014; McCullough et al., 2016).
Full article: Aging Cell
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Archive
- Most read
month
week
day
- Food and Environmental Virology: tea contributes to effective coronavirus control 1165
- Daily Mail: vitamin A, B3 and E supplements can be dangerous 1161
- Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk 1127
- 305 cases of measles recorded in Armenia so far in 2024 1121
- Women are more susceptible to blood loss and death during bypass surgery than men, researchers say 990
- Daily Mail: intermittent fasting is not suitable for children and women before their periods 961
- Diabetes Care: evening physical activity is good for the heart 945
- First Armenian-German Conference entitled “Heart Failure Spring School” 720
- Obesity: exercising before breakfast helps you lose weight faster 637
- Daily Mail: Elderly woman in China gets infected with brain-eating amoeba 625
- The Conversation: childhood trauma can cause pathological hoarding 623
- Why do kids usually recover from COVID-19 more easily than adults? 619
- Daily Mail: Satiating food reduces cravings for sweets, nutritionist says 586
- JAMA Oncology: Urine test can help rule out high-grade prostate cancer with almost 100% accuracy, study shows 502
- Scientists grow human mini-lungs in lab 395
- Find us on Facebook
- Poll





