- Latest news▼
-
18:22, April 17 First Armenian-German Conference entitled “Heart Failure Spring School”

-
14:37, April 16 Daily Mail: intermittent fasting is not suitable for children and women before their periods

-
16:41, April 15 Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk

-
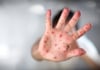
15:04, April 15 305 cases of measles recorded in Armenia so far in 2024
-
14:38, April 15 Food and Environmental Virology: tea contributes to effective coronavirus control

-
12:41, April 15 Daily Mail: vitamin A, B3 and E supplements can be dangerous
-
10:56, April 15 Diabetes Care: evening physical activity is good for the heart

-
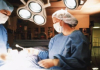
08:27, April 15 Women are more susceptible to blood loss and death during bypass surgery than men, researchers say
-
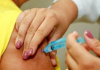
18:42, April 13 WHO: Nigeria pioneers revolutionary meningitis vaccine
-
16:43, April 13 One-third of women experience menstruation-related migraines, most often during premenopause - study

-
14:37, April 12 Pediatrics: Hypoglossal nerve stimulation implant helps with sleep apnea

-
12:13, April 12 Health minister: Simulation educational center will be created, assisted reproductive technology capacity will increase in Armenia

-
17:03, April 11 BMJ: antidepressant injection 4 times reduced risk of severe postpartum depression

-
11:35, April 11 Health minister: Number of hard-to-reach medical services within state funding scope increased in Armenia in 2023

-
16:10, April 10 Breathing therapy, which is so popular in the world, is starting to spread in Armenia

All materials
Safety of Tattoos in Persons Undergoing MRI

Case reports of adverse reactions in persons undergoing magnetic resonance imaging (MRI) have implicated tattoos as a potential source of risk.1-5 Quantitative data are lacking to inform the risk assessment of tattoo-associated adverse events among persons undergoing MRI, but these data are needed given the increasing prevalence of tattoos.
In a prospective study, volunteers at the University College London Wellcome Centre for Human Neuroimaging who were enrolled in neuroimaging studies involving scanners with a magnetic field strength of 3 Tesla were asked to participate if they had at least one tattoo and met specific inclusion criteria (≤5% of the body tattooed, tattoo or tattoos ≤20 cm in length, and no tattoos on the head, neck, or genitals) (see Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). The study was approved by the ethics committee of University College London, and written informed consent was obtained from all the participants.
Characteristics (the number, colors, and dimensions of the tattoos; the country in which the tattoos were applied; and the year of the application of the tattoos) were recorded along with the maximum specific absorption rate of the MRI sequences (which was maintained at <2 watts per kilogram) and the age and sex of the participants. The primary outcome measure was successful completion of MRI. If completion of MRI was unsuccessful, the event was deemed by the investigators to be an adverse reaction if it was tattoo-related.
Between 2011 and 2017, a total of 330 persons who were 18 to 66 years of age underwent MRI in a total of 585 sessions. Five different MRI scanners (one Allegra scanner, three types of Magnetom Trio scanners, and one Magnetom Prisma scanner, all manufactured by Siemens) had either a body coil (in 567 sessions) or a localized head coil (in 18) for radio-frequency transmission. Various types of functional and anatomical imaging were performed.
The participants had one to seven tattoos, and there were a total of 932 unique tattoos across the cohort. Black (in 717 tattoos) was the most common in a range of ink colors (Fig. S1a in the Supplementary Appendix). The maximum tattoo dimension ranged from 1.0 to 20.0 cm (Fig. S1b in the Supplementary Appendix). Tattoos were applied primarily in Europe (570 tattoos), the United Kingdom (456 tattoos), and the Americas (90 tattoos), as well as in Asia, Africa, and Australia. A total of 25 tattoos were applied by the participants themselves. The maximum specific absorption rate of the MRI ranged from 4 to 95%; the median rate was 36% (interquartile range, 29 to 44).
One participant retrospectively reported “awareness” of a tattoo and “tingling” when scanning began. This was not classified by the investigators as a tattoo-related adverse reaction, and it may have been prompted by instructions to the participant to monitor the tattoo location. Another participant, who had several tattoos, reported a warm and tight feeling around one tattoo on the wrist during the localizer sequence, and the MRI was terminated. This reaction was classified by the investigators as a mild tattoo-related adverse reaction. The sensation fully resolved spontaneously over 24 hours without medical intervention. No other tattoo-related adverse reactions were detected.
With the use of the Clopper–Pearson test, the estimated probability of an adverse reaction was 0.17% (95% confidence interval [CI], 0.00 to 0.95) assuming independent observations and 0.30% (95% CI, 0.01 to 1.68) assuming maximal correlation among repeated sessions for individual participants. Our findings indicate a low risk of tattoo-related adverse reactions under these specific study conditions.
New England Journal of Medicine
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Archive
- Most read
month
week
day
- BMJ: antidepressant injection 4 times reduced risk of severe postpartum depression 1410
- Health minister: Number of hard-to-reach medical services within state funding scope increased in Armenia in 2023 1281
- Pediatrics: Hypoglossal nerve stimulation implant helps with sleep apnea 1264
- Health minister: Simulation educational center will be created, assisted reproductive technology capacity will increase in Armenia 1228
- WHO: Nigeria pioneers revolutionary meningitis vaccine 1064
- One-third of women experience menstruation-related migraines, most often during premenopause - study 1040
- Women are more susceptible to blood loss and death during bypass surgery than men, researchers say 727
- Food and Environmental Virology: tea contributes to effective coronavirus control 712
- Daily Mail: vitamin A, B3 and E supplements can be dangerous 709
- 305 cases of measles recorded in Armenia so far in 2024 676
- Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk 672
- Diabetes Care: evening physical activity is good for the heart 668
- Daily Mail: intermittent fasting is not suitable for children and women before their periods 486
- First Armenian-German Conference entitled “Heart Failure Spring School” 241
- Find us on Facebook
- Poll





