- Latest news▼
-
12:16, April 19 Scientists grow human mini-lungs in lab

-
10:23, April 19 JAMA Oncology: Urine test can help rule out high-grade prostate cancer with almost 100% accuracy, study shows

-
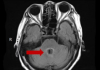
18:00, April 18 Daily Mail: Elderly woman in China gets infected with brain-eating amoeba
-
14:19, April 18 Obesity: exercising before breakfast helps you lose weight faster

-
10:42, April 18 The Conversation: childhood trauma can cause pathological hoarding

-
08:37, April 18 Daily Mail: Satiating food reduces cravings for sweets, nutritionist says

-
18:22, April 17 First Armenian-German Conference entitled “Heart Failure Spring School”

-
08:38, April 17 Why do kids usually recover from COVID-19 more easily than adults?

-
14:37, April 16 Daily Mail: intermittent fasting is not suitable for children and women before their periods

-
16:41, April 15 Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk

-
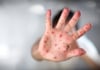
15:04, April 15 305 cases of measles recorded in Armenia so far in 2024
-
14:38, April 15 Food and Environmental Virology: tea contributes to effective coronavirus control

-
12:41, April 15 Daily Mail: vitamin A, B3 and E supplements can be dangerous
-
10:56, April 15 Diabetes Care: evening physical activity is good for the heart

-
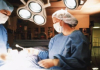
08:27, April 15 Women are more susceptible to blood loss and death during bypass surgery than men, researchers say
All materials
FDA warns Americans against ‘Dracula’ transfusions of young blood
The US Food and Drug Administration (FDA) is warning against getting the 'young blood' transfusions that have become a hot topic in experimental treatments to block aging and Alzheimer's disease.
A number of recent studies on both animals and humans have suggested that compounds in younger people's blood may help treat conditions of old age, including Parkinson's and aging itself.
Some clinics offering the controversial transfusions have cropped up already in the US and abroad.
But the FDA warned today that there are no proven - much less approved - benefits of younger donor blood, and that the procedure's safety has not been established.
A handful of experiments, studies and his own intuition were enough to convince Dr Jesse Karmazin that young-blood might be a cure-all.
And he had already convinced quite a following, opening Ambrosia clinics for young blood transfusions in Los Angeles and San Francisco, California, Tampa, Florida and Omaha, Nebraska.
Ambrosia offered a liter of young blood - or, more accurately, plasma, a component of blood - for $8,000 a bag, but has now shuttered its clinics 'in compliance with the FDA announcement,' a brief statement on the company's website says.
Blood plasma itself is approved for transfusions and is life-saving for people who have lost a lot of blood, have liver disease or have had heart surgery and are low on certain critical blood proteins.
These proteins help the blood clot, which, in turn helps to stop excessive bleeding.
But they are not approved for use in anti-aging (or Alzheimer's or Parkinson's diseases).
With age the volume and quality of our blood does decline, so our levels of blood proteins do, too.
Researchers at Stanford University have shown some promising anti-aging and Alzheimer's-reversing effects when they injected blood from young mice into older ones.
But they're still trying to work out what proteins or parts of those blood transfusions are doing what in the older mice.
And that stage of research comes far before human transfusions, like Ambrosia is already doing.
The FDA warned that not only are the benefits unproven, but that the risks of young blood transfusions may be significant.
'Reports we’re seeing indicate that the dosing of these infusions can involve administration of large volumes of plasma that can be associated with significant risks including infectious, allergic, respiratory and cardiovascular risks, among others,' said FDA Commissioner Dr Scott Gottlieb in a statement.
'Simply put, we’re concerned that some patients are being preyed upon by unscrupulous actors touting treatments of plasma from young donors as cures and remedies.
'Such treatments have no proven clinical benefits for the uses for which these clinics are advertising them and are potentially harmful. There are reports of bad actors charging thousands of dollars for infusions that are unproven and not guided by evidence from adequate and well-controlled trials.'
Source: dailymail.co.uk
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Archive
- Most read
month
week
day
- WHO: Nigeria pioneers revolutionary meningitis vaccine 1180
- One-third of women experience menstruation-related migraines, most often during premenopause - study 1163
- Daily Mail: vitamin A, B3 and E supplements can be dangerous 1003
- Food and Environmental Virology: tea contributes to effective coronavirus control 1000
- Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk 972
- 305 cases of measles recorded in Armenia so far in 2024 964
- Women are more susceptible to blood loss and death during bypass surgery than men, researchers say 962
- Diabetes Care: evening physical activity is good for the heart 922
- Daily Mail: intermittent fasting is not suitable for children and women before their periods 781
- First Armenian-German Conference entitled “Heart Failure Spring School” 565
- Why do kids usually recover from COVID-19 more easily than adults? 449
- The Conversation: childhood trauma can cause pathological hoarding 447
- Obesity: exercising before breakfast helps you lose weight faster 447
- Daily Mail: Elderly woman in China gets infected with brain-eating amoeba 411
- Daily Mail: Satiating food reduces cravings for sweets, nutritionist says 408
- Find us on Facebook
- Poll





