- Latest news▼
-
15:32, April 26 ESCMID: New method of purifying the air with ultraviolet light could protect world from new pandemic

-
08:43, April 26 Enzymes that convert different blood groups into first group are discovered

-
19:41, April 25 Children’s Hospital Los Angeles and International Center of Professional Development Allergy/Immunology Conference

-
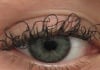
17:31, April 25 JAMA: patient grew long, curly eyelashes because of chemotherapy
-
11:08, April 25 Mpox epidemic declared in Republic of the Congo

-
08:31, April 25 OU: quitting smoking 4 times more likely to cure laryngeal cancer

-
01:20, April 25 Paralyzed man in China writes hieroglyphs using neural implants placed in his brain

-
15:11, April 24 Zombie deer disease possibly linked to hunters’ deaths

-
12:27, April 23 Appetite: Scientists found out the secret to the appeal of large portions of fast food

-
10:33, April 23 Scientists test new approach to fighting viruses

-
08:38, April 23 Ketamine may help with postpartum depression

-
22:12, April 22 Unhealthy amount of sugar found in baby food products of a well-known brand

-
19:41, April 22 Air pollution puts health of more than 1.6 billion workers globally at risk

-
17:25, April 22 Scientists found baked goods and lack of sleep to be more dangerous than alcohol

-
16:02, April 22 342 cases of measles recorded in Armenia so far in 2024
All materials
Evidence that RNA Viruses Drove Adaptive Introgression between Neanderthals and Modern Humans

Neanderthals and modern humans interbred at least twice in the past 100,000 years. While there is evidence that most introgressed DNA segments from Neanderthals to modern humans were removed by purifying selection, less is known about the adaptive nature of introgressed sequences that were retained. We hypothesized that interbreeding between Neanderthals and modern humans led to (1) the exposure of each species to novel viruses and (2) the exchange of adaptive alleles that provided resistance against these viruses. Here, we find that long, frequent—and more likely adaptive—segments of Neanderthal ancestry in modern humans are enriched for proteins that interact with viruses (VIPs). We found that VIPs that interact specifically with RNA viruses were more likely to belong to introgressed segments in modern Europeans. Our results show that retained segments of Neanderthal ancestry can be used to detect ancient epidemics.
After their divergence 500,000 to 800,000 years ago, modern humans and Neanderthals interbred at least twice: the first time ∼100,000 years ago (Kuhlwilm et al., 2016) and the second ∼50,000 years ago (Fu et al., 2015, Green et al., 2010, Pääbo, 2015, Sankararaman et al., 2012, Sankararaman et al., 2014). The first interbreeding episode left introgressed segments (IS) of modern human ancestry within Neanderthal genomes (Kuhlwilm et al., 2016), as revealed by the analysis of ancient DNA from a single Altai Neanderthal individual sequenced by Prüfer et al. (2014). This first interbreeding event appears not to have left any detectable segments of Neanderthal ancestry in extant modern human genomes (Kuhlwilm et al., 2016). In contrast, the second interbreeding episode left detectable IS of Neanderthal ancestry within the genomes of non-African modern humans (Fu et al., 2015, Green et al., 2010, Prüfer et al., 2014, Sankararaman et al., 2014, Vernot and Akey, 2014).
Recent advances in the detection of introgression have led to the discovery that the majority of genomic segments initially introgressed from Neanderthals to modern humans were rapidly removed by purifying selection. Harris and Nielsen (2016) estimated that the proportion of Neanderthal ancestry in modern human genomes rapidly fell from ∼10% to the current levels of 2%–3% in modern Asians and Europeans (Fu et al., 2015, Juric et al., 2016).
This history of interbreeding and purifying selection against IS raises several important questions. First, among the introgressed sequences that were ultimately retained, can we detect which sequences persisted by chance because they were not as deleterious or not deleterious at all to the recipient species, and which persisted not despite natural selection but because of it—that is, which IS increased in frequency due to positive selection? If any of the introgressed sequences were indeed driven into the recipient species due to positive selection, can we determine which pressures in the environment drove this adaptation?
Recently we found that proteins that interact with viruses (virus-interacting proteins [VIPs]) evolve under both stronger purifying selection and tend to adapt at much higher rates compared to similar proteins that do not interact with viruses (Enard et al., 2016).
We estimated that interactions with viruses accounted for ∼30% of protein adaptation in the human lineage (Enard et al., 2016). Because viruses appear to have driven so much adaptation in the human lineage, and because it is plausible that when Neanderthals and modern humans interbred they also exchanged viruses either directly by contact or via their shared environment, we hypothesized that some introgressed sequences might have provided a measure of protection against the exchanged viruses and were driven into the recipient species by positive directional selection. Consistent with this model, several cases of likely adaptive introgression (Gittelman et al., 2016, Racimo et al., 2015, Racimo et al., 2017) from Neanderthals to modern humans involve immune genes that are specialized to deal with pathogens including viruses (Abi-Rached et al., 2011, Dannemann et al., 2016, Deschamps et al., 2016, Houldcroft and Underdown, 2016, Mendez et al., 2012, Mendez et al., 2013, Nedelec et al., 2016, Quach et al., 2016, Sams et al., 2016).
Here, we test this hypothesis by assessing whether VIPs are enriched in IS overall and, more specifically, in longer and more frequent IS that are more likely to have been driven into the recipient genome by positive directional selection. Because purifying selection strongly affects the probability of introgressed sequences being retained by chance, we test introgression enrichments at VIPs after controlling for the stronger purifying selection at VIPs as well as many other potentially confounding factors.
The basic logic of the analysis is as follows. If positive directional selection occurs soon after interbreeding, adaptive Neanderthal introgressed haplotypes are expected to rapidly increase in frequency before being fragmented by recombination and thus should lead to the presence of long and frequent IS as a result. Over time, recombination is expected to break up IS while purifying selection should remove deleterious alleles that hitchhiked together with the adaptive variant(s). As a result, the signal should erode over time.
In summary, we confirmed that the 4,534 VIPs in our set are more conserved and have more segregating deleterious variants than non-VIPs (Enard et al., 2016). The higher levels of conservation and purifying selection of VIPs, and higher loss rate of more constrained sequences of Neanderthal ancestry from the modern human genomes, implies that IS containing VIPs are more likely to have been removed by purifying selection. It is thus essential to control for varying levels of purifying selection to increase the power of detection of enrichment of VIPs in IS. An imperfect control for purifying selection is indeed likely to make the test of the enrichment of VIPs in the introgressed regions overly conservative, as under the null hypothesis of no adaptation preferentially targeting VIPs, we would expect VIPs to be present in the Neanderthal IS less often compared to non-VIPs.
Although we combined LT and HT-VIPs into a single VIP category, throughout the paper, we systematically confirmed that the major results obtained when combining all VIPs also held true when using only the hand-curated LT-VIPs.
Controlling for Confounding Factors between VIPs and Non-VIPs
To determine if VIPs are enriched in segments introgressed between modern humans and Neanderthals, it is important to first define which other factors, in addition to the levels of constraint, affect the occurrence of IS along the genome independently of interactions with viruses. In the genome, factors that affect the occurrence of IS should differ inside compared to outside IS. We must therefore match VIPs and non-VIPs for genomic factors that (1) differ inside versus outside IS, and (2) also differ between VIPs and non-VIPs
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Children’s Hospital Los Angeles and International Center of Professional Development Allergy/Immunology Conference
- First Armenian-German Conference entitled “Heart Failure Spring School”
- Allogeneic bone marrow transplant in case of hematological malignancy performed in Armenia for first time
All materials
- Archive
- Most read
month
week
day
- Scientists found baked goods and lack of sleep to be more dangerous than alcohol 1059
- Next pandemic likely to be triggered by flu - scientists 1046
- 342 cases of measles recorded in Armenia so far in 2024 978
- Unhealthy amount of sugar found in baby food products of a well-known brand 936
- Air pollution puts health of more than 1.6 billion workers globally at risk 926
- Ketamine may help with postpartum depression 917
- Appetite: Scientists found out the secret to the appeal of large portions of fast food 912
- Scientists test new approach to fighting viruses 892
- Zombie deer disease possibly linked to hunters’ deaths 857
- Scientists develop new method to safely stimulate immune cells to fight cancer 842
- Cognitively stimulating jobs in midlife could lower dementia risk in old age, study finds 837
- Blood test can determine who is at risk of developing multiple sclerosis - scientists 835
- BrainStimulation: electrical brain stimulation alleviates anxiety and depression in the elderly 770
- Mpox epidemic declared in Republic of the Congo 654
- Paralyzed man in China writes hieroglyphs using neural implants placed in his brain 630
- Find us on Facebook
- Poll





