- Latest news▼
-
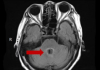
18:00, April 18 Daily Mail: Elderly woman in China gets infected with brain-eating amoeba
-
14:19, April 18 Obesity: exercising before breakfast helps you lose weight faster

-
10:42, April 18 The Conversation: childhood trauma can cause pathological hoarding

-
08:37, April 18 Daily Mail: Satiating food reduces cravings for sweets, nutritionist says

-
18:22, April 17 First Armenian-German Conference entitled “Heart Failure Spring School”

-
08:38, April 17 Why do kids usually recover from COVID-19 more easily than adults?

-
14:37, April 16 Daily Mail: intermittent fasting is not suitable for children and women before their periods

-
16:41, April 15 Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk

-
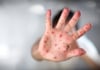
15:04, April 15 305 cases of measles recorded in Armenia so far in 2024
-
14:38, April 15 Food and Environmental Virology: tea contributes to effective coronavirus control

-
12:41, April 15 Daily Mail: vitamin A, B3 and E supplements can be dangerous
-
10:56, April 15 Diabetes Care: evening physical activity is good for the heart

-
08:27, April 15 Women are more susceptible to blood loss and death during bypass surgery than men, researchers say
-
18:42, April 13 WHO: Nigeria pioneers revolutionary meningitis vaccine
-
16:43, April 13 One-third of women experience menstruation-related migraines, most often during premenopause - study

All materials
Alzheimer's drug trial in U.S. results in death of patient

A 65-year-old American woman died of a massive brain hemorrhage during an experimental treatment with a drug called lecanemab for Alzheimer's disease. The drug lecanemab, which is an antibody, is being developed by Biogen in collaboration with the Japanese pharmaceutical company Eisai.
According to Science, the woman received injections as part of a clinical trial and suffered a stroke and bleeding that had previously been seen with similar antibodies. After the stroke in the emergency room at Northwestern University Medical Center in Chicago, she was prescribed the drug to break up the blood clots. However, she could not be saved; the woman died a few days later.
In September, Eisai and Biogen reported positive results from a phase III clinical trial of lekanemab. According to the results, after 18 months from the beginning of the study among volunteers with moderate cognitive impairment and early stage dementia, the drug showed a 27% reduction in cognitive impairment compared to the control group.
Biogen shares fell 5.35% to $288.88 a share in premarket trading on Nov. 28 following the news of the patient's death, NASDAQ trading data showed. Biogen's stock rebounded after the U.S. market opened the session and was trading at $299.19 a share as of 5:41 p.m. Moscow time.
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Archive
- Most read
month
week
day
- Pediatrics: Hypoglossal nerve stimulation implant helps with sleep apnea 1353
- Health minister: Simulation educational center will be created, assisted reproductive technology capacity will increase in Armenia 1311
- WHO: Nigeria pioneers revolutionary meningitis vaccine 1164
- One-third of women experience menstruation-related migraines, most often during premenopause - study 1132
- Women are more susceptible to blood loss and death during bypass surgery than men, researchers say 893
- Daily Mail: vitamin A, B3 and E supplements can be dangerous 872
- Food and Environmental Virology: tea contributes to effective coronavirus control 872
- Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk 841
- 305 cases of measles recorded in Armenia so far in 2024 835
- Diabetes Care: evening physical activity is good for the heart 824
- Daily Mail: intermittent fasting is not suitable for children and women before their periods 649
- First Armenian-German Conference entitled “Heart Failure Spring School” 438
- Why do kids usually recover from COVID-19 more easily than adults? 303
- Obesity: exercising before breakfast helps you lose weight faster 275
- The Conversation: childhood trauma can cause pathological hoarding 270
- Find us on Facebook
- Poll





