- Latest news▼
-
15:32, April 26 ESCMID: New method of purifying the air with ultraviolet light could protect world from new pandemic

-
08:43, April 26 Enzymes that convert different blood groups into first group are discovered

-
19:41, April 25 Children’s Hospital Los Angeles and International Center of Professional Development Allergy/Immunology Conference

-
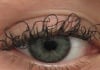
17:31, April 25 JAMA: patient grew long, curly eyelashes because of chemotherapy
-
11:08, April 25 Mpox epidemic declared in Republic of the Congo

-
08:31, April 25 OU: quitting smoking 4 times more likely to cure laryngeal cancer

-
01:20, April 25 Paralyzed man in China writes hieroglyphs using neural implants placed in his brain

-
15:11, April 24 Zombie deer disease possibly linked to hunters’ deaths

-
12:27, April 23 Appetite: Scientists found out the secret to the appeal of large portions of fast food

-
10:33, April 23 Scientists test new approach to fighting viruses

-
08:38, April 23 Ketamine may help with postpartum depression

-
22:12, April 22 Unhealthy amount of sugar found in baby food products of a well-known brand

-
19:41, April 22 Air pollution puts health of more than 1.6 billion workers globally at risk

-
17:25, April 22 Scientists found baked goods and lack of sleep to be more dangerous than alcohol

-
16:02, April 22 342 cases of measles recorded in Armenia so far in 2024
All materials
Girl, 9, battling fatal ‘dementia-like disease’ will unlearn everything she knows ‘after drug trial is stopped’

A LITTLE girl battling “childhood dementia” has been handed a “death sentence”, her parents claim, after a drug trial was stopped without warning.
Tillie Mae Mawdsley spent six years on a clinical trial which her parents say stalled the progression of the extremely rare – and incurable – Sanfilippo disease.
The nine-year-old was diagnosed with the condition, likened to a form of childhood dementia, as a toddler when her family was told to “go home and make memories”.
The degenerative disorder is caused by a missing enzyme and creates a build up of sugar around the muscles, stiffening the joints and resulting in early death.
It affects only a handful of children in the world including Tillie Mae who is now unable to talk and is slowly becoming less mobile every day.
Mum Michala Mawdsley, 36, said: “We feel like the rug has been pulled from beneath us and she’s been handed a life sentence.
“It’s difficult to accept there’s nothing we can do for her now.
“Tillie’s treatment gave her a quality of life.
“The trial was making her better and I believe that drug was working.
“It was a huge risk for us to take but I felt like I was doing something. I’m grateful that we were part of it.”
Tillie Mae was one of six children in the UK offered the enzyme replacement therapy (ERT) by Shire, an American pharmaceutical company.
Shire stated that they didn’t find any improvement in patients’ condition.
But Tillie Mae’s family are adamant the drug stopped her symptoms from worsening.
The treatment involved her making a 400-mile round trip from her home in Hatfield, Hertforshire, to Manchester Children’s Hospital every four weeks.
Without treatment, children with Sanfilippo syndrome regress back to being a toddler as they lose the all the skills learned in early life.
Sufferers are not expected to live past 13 years old and lose the ability to move, swallow and eventually, breathe.
However, Michala and her husband Paul, 40, believe their daughter’s symptoms were stalled thanks to the revolutionary drug.
Michaela said: “We were grateful that she had the opportunity but she stayed the same and that was enough for us.
“They wanted to see a change in children and argued the drug wasn’t causing any cognitive improvement.
“They believed it was going to be the next big thing but then one day the trial just stopped and they never really gave us any real reasons why.
“When Tillie Mae’s sister Lexie found out that the medicine had stopped she said ‘Tillie Mae is going to die now’, it was awful hearing it.
“Being part of something made us feel like we were going somewhere so it’s been a massive blow but we have to be strong for Tillie Mae.”
Tillie Mae has no understanding of what is happening to her because of the condition which is likened to a form of childhood dementia.
“It’s memory loss, the disease attacks the brain,” said Michala, who works as a part-time customer services assistant.
“She is doing extremely well but the disease has got her.
“It’s horrible, it really is like a childhood dementia.
“She’s got everything to live for and a right to live but just because it’s rare, there is no cure.”
Michala and utilities manager Paul, also parents to 10-year-old Lexie, are desperate to raise awareness of the condition – and funds to try and find a cure.
They are hoping to set up a charity to raise the profile of Sanfilippo syndrome help aid future research.
Michala said: “We need to raise the funds and raise awareness.
“We want something here for children suffering in the UK.
“It would be a dream to find a cure for Sanfilippo.
“People need to know what it is just like they know what meningitis and Down’s Syndrome is.
“For now we’re continuing to make memories and just living day by day, taking each day as it comes before Tillie Mae runs out of time.
“Hopefully in time we can help to find a cure.”
A spokesman for Shire said: “Shire is extremely disappointed to have announced that this Phase 2b study did not meet its primary endpoint of slowing the cognitive decline in patients.
“Analysis of secondary endpoints and exploratory endpoints, which were designed to further examine whether there was a therapeutic effect on slowing cognitive decline, an effect on behavioural achievements and slowing the loss of brain volume, were also not met.
“The results did not show any slowing of disease progression in these patients compared to control (untreated) patients.
“Given the lack of efficacy seen in the results of this study, Shire made the decision to close the MPS IIIA program, which includes discontinuing any ongoing extension studies.
“MPS IIIA is a devastating disease for which there are currently no approved treatment options.
“We are disappointed for and sympathetic to the Sanfilippo community and thank all the patients and their families for their willingness to participate in these trials.”
To support Tillie Mae, visit her GoFundMe page here.
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Children’s Hospital Los Angeles and International Center of Professional Development Allergy/Immunology Conference
- First Armenian-German Conference entitled “Heart Failure Spring School”
- Allogeneic bone marrow transplant in case of hematological malignancy performed in Armenia for first time
All materials
- Archive
- Most read
month
week
day
- Scientists found baked goods and lack of sleep to be more dangerous than alcohol 1054
- Next pandemic likely to be triggered by flu - scientists 1040
- 342 cases of measles recorded in Armenia so far in 2024 974
- Unhealthy amount of sugar found in baby food products of a well-known brand 912
- Air pollution puts health of more than 1.6 billion workers globally at risk 903
- Ketamine may help with postpartum depression 896
- Appetite: Scientists found out the secret to the appeal of large portions of fast food 890
- Scientists test new approach to fighting viruses 870
- Scientists develop new method to safely stimulate immune cells to fight cancer 837
- Zombie deer disease possibly linked to hunters’ deaths 833
- Cognitively stimulating jobs in midlife could lower dementia risk in old age, study finds 833
- Blood test can determine who is at risk of developing multiple sclerosis - scientists 831
- BrainStimulation: electrical brain stimulation alleviates anxiety and depression in the elderly 767
- Mpox epidemic declared in Republic of the Congo 632
- Paralyzed man in China writes hieroglyphs using neural implants placed in his brain 607
- Find us on Facebook
- Poll





