- Latest news▼
-
17:25, April 22 Scientists found baked goods and lack of sleep to be more dangerous than alcohol

-
16:02, April 22 342 cases of measles recorded in Armenia so far in 2024
-
15:29, April 22 BrainStimulation: electrical brain stimulation alleviates anxiety and depression in the elderly

-
08:27, April 22 Cognitively stimulating jobs in midlife could lower dementia risk in old age, study finds

-
20:37, April 21 Environmental Health Perspectives: Microplastics ingested with food and water can spread from the gut to the brain

-
22:41, April 20 Scientists develop new method to safely stimulate immune cells to fight cancer

-
20:46, April 20 Blood test can determine who is at risk of developing multiple sclerosis - scientists

-
18:36, April 20 Next pandemic likely to be triggered by flu - scientists

-
12:16, April 19 Scientists grow human mini-lungs in lab

-
10:23, April 19 JAMA Oncology: Urine test can help rule out high-grade prostate cancer with almost 100% accuracy, study shows

-
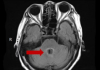
18:00, April 18 Daily Mail: Elderly woman in China gets infected with brain-eating amoeba
-
14:19, April 18 Obesity: exercising before breakfast helps you lose weight faster

-
10:42, April 18 The Conversation: childhood trauma can cause pathological hoarding

-
08:37, April 18 Daily Mail: Satiating food reduces cravings for sweets, nutritionist says

-
18:22, April 17 First Armenian-German Conference entitled “Heart Failure Spring School”

All materials
3 incredible medical breakthroughs in 2022

Scientists have made breakthroughs in 2022 that will pave the way for new drugs and vaccines to treat serious diseases that are virtually untreatable.
Let's talk about three of the most important breakthroughs of 2022.
1.RSV vaccine shows promise for first time in 50 years
Two RSV vaccines could be approved by the end of 2023 after nearly 50 years of no meaningful progress, according to the creators of the two vaccines.
RSV usually causes mild, flu-like symptoms that go away within a few weeks without treatment, according to the Centers for Disease Control and Prevention.
But the virus can be serious, especially for infants and the elderly, because it can cause pneumonia and inflammation of the small airways, called bronchiolitis, which can lead to respiratory failure and death. According to the CDC, more than a hundred infants and 10,000 adults over the age of 65 die from RSV each year in the United States. According to Insider, RSV struck the U.S. earlier this year than usual, with both toddlers and school-age children falling ill.
There is no approved vaccine or treatment for RSV. Moreover, in the late 1960s, the RSV vaccine exacerbated symptoms in children and infants.
Two new vaccines, aimed at pregnant women and the elderly, respectively, target a part of the virus called the fusion protein, which attaches to cells before infecting them. Vaccines that exacerbated the disease were aimed at its subsequent attachment.
GSK said its vaccine for people over 60 was about 94% effective in preventing severe disease in trials involving about 25,000 people.
Pfizer said its vaccine for pregnant women who transmit antibodies to their baby was about 69% effective in preventing severe RSV in newborns during the first six months of life in trials involving about 7,400 people.
Dr. Wilbur Chen, an infectious disease expert at the University of Maryland School of Medicine who researches vaccines and is an independent advisor to the CDC, previously told Insider that if the vaccines are approved for use in the United States, over time we will see a significant reduction in hospitalizations and deaths from RSV.
2.Scientists cured aggressive blood cancer with gene editing
Scientists have cured the world's first aggressive blood cancer using gene editing technology, which was invented just six years ago.
A 13-year-old patient named Alyssa was diagnosed with T-cell acute lymphoblastic leukemia in May 2021, after what her family thought was a period of colds, viruses and general fatigue.
With T-ALL, the body produces too many white blood cells, such as T cells, which don't work as well against infections as normal T cells do. Doctors initially treated Alyssa with chemotherapy and a bone marrow transplant, but the disease returned.
In a clinical trial in May, researchers at Great Ormond Street Hospital in the United Kingdom treated Alyssa with T cells from a healthy donor that had been genetically altered and then gave her another bone marrow transplant.
Our DNA, or genetic code, is made up of individual letters called bases. Researchers altered the donor T cells by chemically transforming the individual letters, which is called base-editing.
The scientists changed the code so that Alyssa's immune system and other cancer therapies could no longer attack the donor T cells, and the modified T cells could destroy the cancerous T cells but not themselves.
According to GOSH, 28 days after the experimental treatment, Alyssa's disease reached remission.
3.Alzheimer's drug slowed the progression of the disease
An experimental drug slowed the progression of Alzheimer's disease in a study that ended decades of failed research and which experts hope shows that new drugs to treat Alzheimer's disease, the most common form of dementia, are possible.
Existing treatments for Alzheimer's help control symptoms, but cannot reverse or cure the disease.
In the study, 898 people between the ages of 50 and 90 who received the drug slowed cognitive decline by about a quarter over 18 months of treatment, compared with 897 people who were given the dummy drug by scientists.
The drug, called lecanemab, is a monoclonal antibody -- a lab-created version of proteins that the body produces to attack viruses or bacteria -- that has been designed to force the immune system to clear amyloid protein from the brain.
John Hardy, the world's leading neurogeneticist at the University of California, U.K., who discovered the mutation that led to the amyloid hypothesis about 30 years ago, told BBC News that the findings are historic.
The bi-weekly infusion may slow the progression of the disease, but it is not a miracle cure, and there are risks involved.
Overall, nearly 7 percent of people who received the drug had to stop taking it because of side effects, and we don't know about its effects after 18 months.
The FDA is currently evaluating the data to decide whether to approve lekanemab for widespread use starting in 2023.
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Archive
- Most read
month
week
day
- Daily Mail: intermittent fasting is not suitable for children and women before their periods 1298
- JAMA Oncology: Urine test can help rule out high-grade prostate cancer with almost 100% accuracy, study shows 1110
- Daily Mail: Elderly woman in China gets infected with brain-eating amoeba 1102
- Obesity: exercising before breakfast helps you lose weight faster 1092
- The Conversation: childhood trauma can cause pathological hoarding 1090
- Daily Mail: Satiating food reduces cravings for sweets, nutritionist says 1068
- First Armenian-German Conference entitled “Heart Failure Spring School” 1045
- Why do kids usually recover from COVID-19 more easily than adults? 985
- Scientists grow human mini-lungs in lab 921
- Next pandemic likely to be triggered by flu - scientists 450
- Scientists found baked goods and lack of sleep to be more dangerous than alcohol 397
- Blood test can determine who is at risk of developing multiple sclerosis - scientists 370
- Scientists develop new method to safely stimulate immune cells to fight cancer 356
- 342 cases of measles recorded in Armenia so far in 2024 338
- Cognitively stimulating jobs in midlife could lower dementia risk in old age, study finds 337
- Find us on Facebook
- Poll





