- Latest news▼
-
18:15, May 4 What role does diet plays in the treatment of bipolar disorder?

-
10:52, May 4 Scientists identified dangerous health effects for families with gas cookers in their homes

-
12:36, May 3 Smoking during pregnancy may lead to obese children, study finds

-
10:29, May 3 Scientists discover cell responsible for repairing damaged liver tissue

-
20:17, May 2 NBR: low to moderate intensity exercise protects against depression

-
18:28, May 2 Sixth Armenian International Ophthalmological Conference will be held on 17-19 May

-
16:11, May 2 ВJN: High-fat foods may increase the risk of heart attack and stroke at a young age

-
14:16, May 2 Patterns: Neural network created that models outcome of various patient therapy methods

-
12:05, May 2 Armenia joins Council of Europe convention on protection of patients' rights

-
14:27, May 1 Journal of Neuroscience: Sluggishness of the elderly is due to greater use of calories, research finds

-
10:26, May 1 AstraZeneca admits its Covid vaccine can cause thrombosis
-
19:31, April 30 Scientists calculated how much time per day you should be sitting, standing and sleeping

-
17:37, April 30 The Conversation: Keeping bread in the fridge improves its health benefits

-
14:30, April 30 Frontiers in Molecular Biosciences: Researchers use AI to identify 191 new destructive viruses
-
08:38, April 30 The Guardian: First personalized cancer vaccine developed

All materials
The first case of gene editing inside the body of a living human
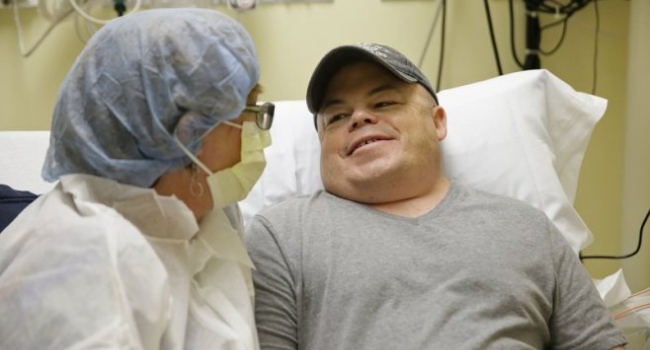 Brian Madeux’s life hasn’t been easy. So far, he’s had 26 operations to fix problems in everything from hernias to eyes. He has a rare disease called Hunter syndrome, which is caused by the lack of a gene that’s used to produce an enzyme that breaks down certain carbohydrates. As a result, the carbohydrates build up in his body’s cells causing all sorts of problems.
Brian Madeux’s life hasn’t been easy. So far, he’s had 26 operations to fix problems in everything from hernias to eyes. He has a rare disease called Hunter syndrome, which is caused by the lack of a gene that’s used to produce an enzyme that breaks down certain carbohydrates. As a result, the carbohydrates build up in his body’s cells causing all sorts of problems.
There is no cure. One way to deal with some of the symptoms is to receive regular doses of the missing enzyme, which may cost him in the US between $100,000 and $400,000 per year. Even then, the enzyme won’t reverse the damage made already and it won’t stop further deterioration that happens in the brain.
But Madeux’s life might be about to change. He is the first patient to receive an experimental gene therapy as part of a clinical trial. Earlier this week, Sangamo Therapeutics injected Madeux with viruses containing a package of gene-editing material, according to the AP. The hope is that these viruses will enter Madeux’s cells, specifically liver cells, inject the missing gene at the right place in his DNA. Only about 1% of the liver’s cells need to be fixed, and give his liver the ability to produce the enzyme he has been missing all his life.
Previously, scientists have extracted human immune cells, then edited the genes in them and put them back as a means of giving those cells the ability to attack lung cancer. And it has worked. If Madeux’s treatment is successful, it would be the first time a gene therapy has worked from inside the human body.
To achieve the gene editing, Sangamo is using a technology called zinc-finger nucleases. Like Crispr, it’s a tool that has the ability to locate the precise location in the 3-billion-letter long strands of DNA and make a snip. Along with the zinc-finger nucleases, the virus also contains snippets of the missing gene, which is then inserted by the body’s own gene-repairing system into the place where the cut is made.
What makes gene-editing different from any other treatment is that it’s not reversible. Once the gene is part of a person’s DNA, it will be with them forever. As part of an approved clinical trial, the therapy has gone through rigorous tests to make the process as safe as possible. The virus, for instance, is built in a such a way that it can’t enter sperm or egg cells, and hence won’t be passed down to the next generation, if the patient were to ever have children.
But there’s no guarantee the treatment will work. Past gene therapy trials have ended in tragedy, where although inserting genes have helped alleviate the problem, the tool also injected genes into unintended parts of the DNA, which caused harm. Tools like zinc-finger nucleases and Crispr promise to get rid of the unintended consequences.
Not more than 10,000 people have Madeux’s kind of metabolic disease, because many don’t live on to become adults. If the trial is successful, Sangamo hopes to treat children with these conditions. This way, the treatment will be given before there’s significant damage to the body. It’ll also be a big boost for gene editing, opening up the potential for treating diseases that have remained incurable until now.
We’ll know in a month’s time whether Madeux’s therapy is working as hoped, and it’ll take as much as three months to be certain. “I’m nervous and excited,” Madeux told the AP. “I’ve been waiting for this my whole life, something that can potentially cure me.”
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Archive
- Most read
month
week
day
- 3 women diagnosed with HIV for first time after getting ‘beauty injections’ in US 1259
- The Guardian: First personalized cancer vaccine developed 1137
- AstraZeneca admits its Covid vaccine can cause thrombosis 1115
- Scientists calculated how much time per day you should be sitting, standing and sleeping 1111
- In 64% of COVID-19 survivors, the condition worsens after one year 1109
- The Conversation: Keeping bread in the fridge improves its health benefits 1096
- Ketodiet improved the condition of patients with schizophrenia and bipolar disorder 1018
- Frontiers in Molecular Biosciences: Researchers use AI to identify 191 new destructive viruses 1012
- 362 cases of measles recorded in Armenia so far in 2024 938
- Journal of Neuroscience: Sluggishness of the elderly is due to greater use of calories, research finds 932
- ECU: body tilt in patients after stroke was found to be curable 883
- Sixth Armenian International Ophthalmological Conference will be held on 17-19 May 863
- Blood: blood cancer drugs may be useful in rheumatoid arthritis 850
- Armenia joins Council of Europe convention on protection of patients' rights 750
- NBR: low to moderate intensity exercise protects against depression 743
- Find us on Facebook
- Poll





