- Latest news▼
-
15:32, April 26 ESCMID: New method of purifying the air with ultraviolet light could protect world from new pandemic

-
08:43, April 26 Enzymes that convert different blood groups into first group are discovered

-
19:41, April 25 Children’s Hospital Los Angeles and International Center of Professional Development Allergy/Immunology Conference

-
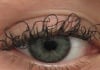
17:31, April 25 JAMA: patient grew long, curly eyelashes because of chemotherapy
-
11:08, April 25 Mpox epidemic declared in Republic of the Congo

-
08:31, April 25 OU: quitting smoking 4 times more likely to cure laryngeal cancer

-
01:20, April 25 Paralyzed man in China writes hieroglyphs using neural implants placed in his brain

-
15:11, April 24 Zombie deer disease possibly linked to hunters’ deaths

-
12:27, April 23 Appetite: Scientists found out the secret to the appeal of large portions of fast food

-
10:33, April 23 Scientists test new approach to fighting viruses

-
08:38, April 23 Ketamine may help with postpartum depression

-
22:12, April 22 Unhealthy amount of sugar found in baby food products of a well-known brand

-
19:41, April 22 Air pollution puts health of more than 1.6 billion workers globally at risk

-
17:25, April 22 Scientists found baked goods and lack of sleep to be more dangerous than alcohol

-
16:02, April 22 342 cases of measles recorded in Armenia so far in 2024
All materials
A new antibiotic weakness—drugs themselves help bacteria survive
Antibiotics save lives, but they are not fail-safe. Even when microbes haven’t acquired drug-evading genetic mutations—a hallmark of antibiotic resistance—the medications don’t always clear infections. A new study identifies a surprising reason why: At infection sites, antibiotics change the natural mixture of chemicals made by the body in ways that protect infecting bacteria. They also thwart the ability of the host’s immune cells to fight off the intruders.
These findings, published Thursday in Cell Host & Microbe, could help scientists “build more effective treatments,” says James Collins, a biological engineer at Massachusetts Institute of Technology and senior author of the paper. Down the line it may be possible to administer antibiotics along with other substances that either mitigate these changes or have the opposite effect, making drugs more effective, he says.
As The Scientific American reports, Collins and his colleagues at MIT’s Broad Institute, Harvard University and the University of California, San Diego, infected mice with Escherichia coli bacteria and gave some of them antibiotics. The scientists then took tissue samples from the mice and analyzed levels of certain bodily chemicals—known as metabolites—that bacteria can use to grow and multiply. At the sites of their infections, mice fed antibiotics had higher levels of some metabolites when compared with drug-free mice. The levels were also higher than what scientists detected in healthy mice.
To see if the metabolites changed antibiotic effectiveness, Collins and his colleagues added these isolated chemicals to E. coli grown in lab plates. They found that they needed to add higher drug concentrations to kill the bacteria when some of the chemicals were present. In other words, the chemicals that had been ramped up in the infected, antibiotic-treated animals were, ironically, making bacteria less susceptible to the drugs.
These chemical changes were incited not by bacterial cells, but by the animals’ own cells. The researchers learned this after giving antibiotics to so-called “germ-free” mice that had no bacteria and saw the same chemical changes. “It really is surprising,” says Eric Brown, the Canada Research Chair in Microbial Chemical Biology at McMaster University in Ontario, who was not involved in the research. “Antibiotics are supposed to be ‘magic bullets’ directed at bacteria but not at the person suffering from infection. Well, this work suggests that there is more going on, on the host side of things, than we might have thought.”
It is unclear how much these changes might reduce antibiotic efficacy in infected people. “We suspect that the strength of this effect will really depend on the type of infection and types of antibiotics used,” says one of the paper co-authors, Jason Yang, a postdoctoral bioengineer at MIT. Some drugs, such as streptomycin, are very sensitive to local chemical changes, he says, so these effects could significantly impair the medication, but more studies are needed.
Collins also is not sure how the chemicals elicit these effects. But he notes some of the compounds slow down aspects of bacterial metabolism, making the antibiotics less lethal. Most antibiotics speed up bacterial metabolism while also de-stabilizing the metabolic process, leading to the build-up of toxic molecules inside the bacteria that help to kill them. With this process dampened, bacteria more easily survive.
It is unlikely the host cells are producing these metabolites as part of a directed reaction, Yang says. “We don't think that this is a programmed biochemical response, that when a host cell sees an infection and sees an antibiotic, that this is what it will produce,” he says. Instead, it is probably that “the nonspecific side effects of these antibiotics in the presence of the bugs are causing other physiological changes that we don’t know about. And these metabolites might be released as kind of by-products.”
Antibiotics also seem to interfere with the infection-fighting activity of host immune cells. When Collins and his team added antibiotics to mouse immune cells called macrophages, the cells consumed less oxygen, necessary for bacteria-killing activity. Then they exposed macrophages that had been treated with antibiotics to E. coli. They found that compared with macrophages that had not been treated with antibiotics those that had been exposed to the drugs engulfed and destroyed fewer bacteria.
Yang stresses that these findings do not mean antibiotics are useless. “Antibiotics work really, really well,” he says. “For the vast majority of infections, [when] we give an antibiotic locally, it will clear the infection.” But the findings do suggest infections are complicated environments, and that antibiotics influence more than just bacterial cells, often in unexpected ways. “That’s one of the really important concepts that I hope we can convey—that it’s complicated, and the complicated parts do matter,” he says.
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Children’s Hospital Los Angeles and International Center of Professional Development Allergy/Immunology Conference
- First Armenian-German Conference entitled “Heart Failure Spring School”
- Allogeneic bone marrow transplant in case of hematological malignancy performed in Armenia for first time
All materials
- Archive
- Most read
month
week
day
- Scientists found baked goods and lack of sleep to be more dangerous than alcohol 1038
- Next pandemic likely to be triggered by flu - scientists 1014
- 342 cases of measles recorded in Armenia so far in 2024 962
- Scientists develop new method to safely stimulate immune cells to fight cancer 821
- Cognitively stimulating jobs in midlife could lower dementia risk in old age, study finds 821
- Blood test can determine who is at risk of developing multiple sclerosis - scientists 816
- Unhealthy amount of sugar found in baby food products of a well-known brand 813
- Air pollution puts health of more than 1.6 billion workers globally at risk 809
- Ketamine may help with postpartum depression 805
- Appetite: Scientists found out the secret to the appeal of large portions of fast food 800
- Scientists test new approach to fighting viruses 782
- BrainStimulation: electrical brain stimulation alleviates anxiety and depression in the elderly 749
- Zombie deer disease possibly linked to hunters’ deaths 725
- Mpox epidemic declared in Republic of the Congo 530
- Paralyzed man in China writes hieroglyphs using neural implants placed in his brain 503
- Find us on Facebook
- Poll





