- Latest news▼
-
15:32, April 26 ESCMID: New method of purifying the air with ultraviolet light could protect world from new pandemic

-
08:43, April 26 Enzymes that convert different blood groups into first group are discovered

-
19:41, April 25 Children’s Hospital Los Angeles and International Center of Professional Development Allergy/Immunology Conference

-
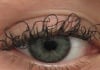
17:31, April 25 JAMA: patient grew long, curly eyelashes because of chemotherapy
-
11:08, April 25 Mpox epidemic declared in Republic of the Congo

-
08:31, April 25 OU: quitting smoking 4 times more likely to cure laryngeal cancer

-
01:20, April 25 Paralyzed man in China writes hieroglyphs using neural implants placed in his brain

-
15:11, April 24 Zombie deer disease possibly linked to hunters’ deaths

-
12:27, April 23 Appetite: Scientists found out the secret to the appeal of large portions of fast food

-
10:33, April 23 Scientists test new approach to fighting viruses

-
08:38, April 23 Ketamine may help with postpartum depression

-
22:12, April 22 Unhealthy amount of sugar found in baby food products of a well-known brand

-
19:41, April 22 Air pollution puts health of more than 1.6 billion workers globally at risk

-
17:25, April 22 Scientists found baked goods and lack of sleep to be more dangerous than alcohol

-
16:02, April 22 342 cases of measles recorded in Armenia so far in 2024
All materials
US regulators wave through Pfizer’s leukaemia drug
The U.S. Food and Drug Administration (FDA) said on Thursday it approved Pfizer Inc's rare blood cancer drug, Besponsa, with a boxed warning.
Besponsa was approved to treat adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL).
Based on the typical duration of treatment, the total cost of the drug will be $168,300, before discounts to purchasers, Pfizer told Reuters.
Besponsa will carry a boxed warning, the severest form of warning by the FDA, saying patients treated with the drug were at a risk of severe liver damage as well as an increased risk of death for patients who take the drug after receiving a certain type of stem cell transplant.
B-cell precursor ALL is a rapidly progressing cancer in which the bone marrow makes too many B-cell lymphocytes, an immature type of white blood cell.
Besposa — approved in the European Union earlier this year — is a targeted therapy that binds to B-cell ALL cancer cells that express the CD22 antigen, blocking the growth of cancer cells.
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Children’s Hospital Los Angeles and International Center of Professional Development Allergy/Immunology Conference
- First Armenian-German Conference entitled “Heart Failure Spring School”
- Allogeneic bone marrow transplant in case of hematological malignancy performed in Armenia for first time
All materials
- Archive
- Most read
month
week
day
- Scientists found baked goods and lack of sleep to be more dangerous than alcohol 1041
- Next pandemic likely to be triggered by flu - scientists 1017
- 342 cases of measles recorded in Armenia so far in 2024 965
- Unhealthy amount of sugar found in baby food products of a well-known brand 836
- Air pollution puts health of more than 1.6 billion workers globally at risk 831
- Ketamine may help with postpartum depression 828
- Scientists develop new method to safely stimulate immune cells to fight cancer 826
- Cognitively stimulating jobs in midlife could lower dementia risk in old age, study finds 824
- Blood test can determine who is at risk of developing multiple sclerosis - scientists 822
- Appetite: Scientists found out the secret to the appeal of large portions of fast food 822
- Scientists test new approach to fighting viruses 803
- BrainStimulation: electrical brain stimulation alleviates anxiety and depression in the elderly 754
- Zombie deer disease possibly linked to hunters’ deaths 751
- Mpox epidemic declared in Republic of the Congo 556
- Paralyzed man in China writes hieroglyphs using neural implants placed in his brain 530
- Find us on Facebook
- Poll





