- Latest news▼
-
12:16, April 19 Scientists grow human mini-lungs in lab

-
10:23, April 19 JAMA Oncology: Urine test can help rule out high-grade prostate cancer with almost 100% accuracy, study shows

-
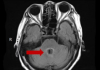
18:00, April 18 Daily Mail: Elderly woman in China gets infected with brain-eating amoeba
-
14:19, April 18 Obesity: exercising before breakfast helps you lose weight faster

-
10:42, April 18 The Conversation: childhood trauma can cause pathological hoarding

-
08:37, April 18 Daily Mail: Satiating food reduces cravings for sweets, nutritionist says

-
18:22, April 17 First Armenian-German Conference entitled “Heart Failure Spring School”

-
08:38, April 17 Why do kids usually recover from COVID-19 more easily than adults?

-
14:37, April 16 Daily Mail: intermittent fasting is not suitable for children and women before their periods

-
16:41, April 15 Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk

-
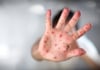
15:04, April 15 305 cases of measles recorded in Armenia so far in 2024
-
14:38, April 15 Food and Environmental Virology: tea contributes to effective coronavirus control

-
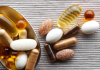
12:41, April 15 Daily Mail: vitamin A, B3 and E supplements can be dangerous
-
10:56, April 15 Diabetes Care: evening physical activity is good for the heart

-
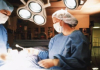
08:27, April 15 Women are more susceptible to blood loss and death during bypass surgery than men, researchers say
All materials
Acute sleep loss results in tissue-specific alterations in genome-wide DNA methylation state and metabolic fuel utilization in humans

Curtailed sleep promotes weight gain and loss of lean mass in humans, although the underlying molecular mechanisms are poorly understood. We investigated the genomic and physiological impact of acute sleep loss in peripheral tissues by obtaining adipose tissue and skeletal muscle after one night of sleep loss and after one full night of sleep. We find that acute sleep loss alters genome-wide DNA methylation in adipose tissue, and unbiased transcriptome-, protein-, and metabolite-level analyses also reveal highly tissue-specific changes that are partially reflected by altered metabolite levels in blood. We observe transcriptomic signatures of inflammation in both tissues following acute sleep loss, but changes involving the circadian clock are evident only in skeletal muscle, and we uncover molecular signatures suggestive of muscle breakdown that contrast with an anabolic adipose tissue signature. Our findings provide insight into how disruption of sleep and circadian rhythms may promote weight gain and sarcopenia.
Chronic sleep loss, social jet lag, and shift work—widespread in our modern 24/7 societies—are associated with an increased risk of numerous metabolic pathologies, including obesity, metabolic syndrome, and type 2 diabetes . Even minor weekly shifts in sleep timing, or as few as five consecutive nights of short sleep, have been associated with an increased risk of weight gain in healthy humans .
Many of the adverse effects attributed to sleep loss and circadian misalignment might arise due to tissue-specific metabolic perturbations in peripheral tissues such as skeletal muscle and adipose tissue. Recurrent sleep loss combined with moderate calorie restriction in humans increases the loss of fat-free body mass, while decreasing the proportion of weight lost as fat, suggesting that sleep loss can promote adverse tissue-specific catabolism and anabolism. Human cohort studies and interventional sleep restriction studies in animals also suggest that sleep loss specifically promotes loss of muscle mass , but the underlying molecular mechanisms remain elusive.
Notably, sleep restriction studies controlling for caloric intake provide evidence that sleep loss reduces the respiratory exchange ratio , indicating a shift toward non-glucose, that is, fatty acid, oxidation. Animal studies have elegantly shown that metabolic fuel selection and overall anabolic versus catabolic homeostasis are regulated by tissue-specific rhythms driven by the core circadian clock. Key metabolic processes, for example, glycolysis and mitochondrial oxidative metabolism, exhibit 24-hour rhythms in tissues such as skeletal muscle. This is, to a significant extent, orchestrated through circadian regulation of key transcription factors and enzymes such as pyruvate dehydrogenase kinase 4 (Pdk4), Ldhb, and phosphofructokinase 2 (Pfk2), which belong to some of the most highly rhythmic transcripts in skeletal muscle across circadian data sets in mice. Correspondingly, ablation of the core clock gene Bmal1 alters metabolic fuel utilization in mice, and circadian desynchrony in humans results in decreased resting metabolic rate. Furthermore, even a single night of sleep loss has been shown to induce tissue-specific transcriptional and DNA methylation (an epigenetic modification that can regulate chromatin structure and gene expression) changes to core circadian clock genes in humans, but the downstream tissue-specific impact on metabolic pathways remains to be determined. Moreover, it is presently unknown to what extent DNA methylation may be modulated throughout the human genome in metabolic tissues in response to acute sleep loss, and whether metabolic tissues respond in a tissue-specific manner across multiple genomic and molecular levels.
On the basis of the above observations, and as a model of shift work that often entails overnight wakefulness, we hypothesized that acute sleep loss (that is, overnight wakefulness) would induce tissue-specific alterations at the genomic and physiological levels in pathways regulating metabolic substrate utilization and anabolic versus catabolic state. Specifically, we expected acute sleep loss to increase non-glycolytic oxidation and protein breakdown in skeletal muscle, with the former favoring hyperglycemia. Since recurrent sleep loss has also been linked to adverse weight gain, we also hypothesized that acute sleep loss would promote signatures of increased adipogenesis and that some of these tissue-specific changes would be reflected at the DNA methylation level, indicating altered “metabolic memory.” To this end, we carried out a range of molecular analyses in subcutaneous adipose tissue and skeletal muscle samples, complemented by analyses in blood, in samples obtained from healthy young men both after a night of sleep loss and after a night of full sleep.
Acute sleep loss results in tissue-specific DNA methylation and transcriptomic changes Whereas the average fold change in methylation for the significant DMRs did not exceed 3% in response to sleep loss (average, +2.8 ± 0.0% and −2.4 ± 0.0% for hyper- and hypomethylated DMRs, respectively), the most highly hypermethylated DMR (on average +6.9%) was found for CD36 , which is involved in fatty acid import and whose expression is dysregulated in obese and type 2 diabetic patients
In contrast to adipose tissue, no significant DMRs were observed in skeletal muscle following sleep loss compared with sleep . This finding could indicate that other epigenetic modifications—that may also respond to environmental changes (for example, at the chromatin level) regulate the transcriptional response to sleep loss in skeletal muscle or, alternatively, that DNA methylation changes occur at, for example, earlier or later time points in our intervention.
Follow NEWS.am Medicine on Facebook and Twitter
- Video
- Event calendar
- Archive
- Most read
month
week
day
- WHO: Nigeria pioneers revolutionary meningitis vaccine 1203
- One-third of women experience menstruation-related migraines, most often during premenopause - study 1201
- Daily Mail: vitamin A, B3 and E supplements can be dangerous 1116
- Food and Environmental Virology: tea contributes to effective coronavirus control 1115
- Cell: in carriers of defective BRCA2 gene, sugar consumption increases cancer risk 1081
- 305 cases of measles recorded in Armenia so far in 2024 1075
- Women are more susceptible to blood loss and death during bypass surgery than men, researchers say 982
- Diabetes Care: evening physical activity is good for the heart 936
- Daily Mail: intermittent fasting is not suitable for children and women before their periods 910
- First Armenian-German Conference entitled “Heart Failure Spring School” 675
- Obesity: exercising before breakfast helps you lose weight faster 581
- The Conversation: childhood trauma can cause pathological hoarding 574
- Why do kids usually recover from COVID-19 more easily than adults? 573
- Daily Mail: Elderly woman in China gets infected with brain-eating amoeba 573
- Daily Mail: Satiating food reduces cravings for sweets, nutritionist says 537
- Find us on Facebook
- Poll





